IXINITY® dosing & administration
Reconstitution Kit: Convenience in preparation.
- Preassembled syringe with plunger attached
- Secure syringe-to-vial connection
- Vacuum seal for ease of drawing diluent into vial
- See the INSTRUCTIONS FOR USE section of the IXINITY package insert for reconstitution, pooling, and administration instructions.
Planet-friendly packaging takes
the patient’s world into account.
IXINITY comes in a fully collapsible, 100% recyclable carton.
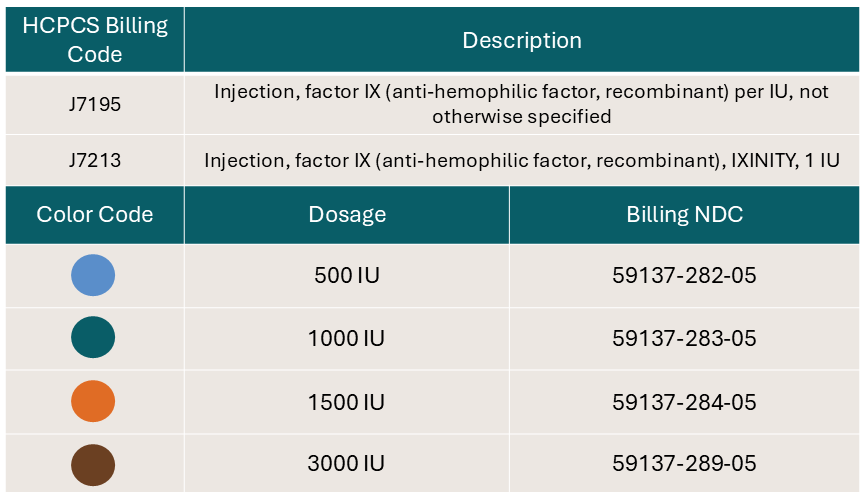
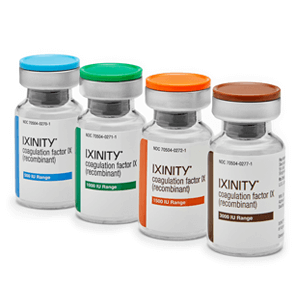
Flexibility of IXINITY vial strengths
IXINITY is available in 500, 1000, 1500, and 3000 IU single-use vials, which are color-coded by vial strength.1

To learn more about how to order IXINITY, call Core Connections at 1-855-IXINITY (1-855-494-6489).
Storage and handling1
- Store at 2°C–25°C (36°F to 77°F)
- May be stored at room temperature or under refrigerated conditions
- 36-month shelf life from date of manufacture
- Do not freeze
- Keep the vial in the carton and protect from light
- Reconstituted solution should be infused immediately or within 3 hours of storage at room temperature after reconstitution
- Do not refrigerate after reconstitution
Custom ancillaries
- Patients choose the supplies they want delivered with IXINITY, such as:
- Butterfly needles
- Bandages, alcohol wipes, gauzes
- Tourniquets
- Delivered in a reusable bag
- Can be delivered to the pharmacy for inclusion in shipment of product
- No cost to patient, prescriber, or pharmacy
- Download form online; NPI signature required

Reference: 1. IXINITY [coagulation factor IX (recombinant)]. Prescribing information. Chicago, IL: Medexus Pharma, Inc.; March 2024.



