
For pediatric patients under 12 years of age
Go low with IXINITY® to keep factor IX coverage high
Proven bleed prevention and control
IXINITY delivers durable prophylactic efficacy
IXINITY gives healthcare professionals the ability to tailor administration schedules to their pediatric patients’ peak activity periods.

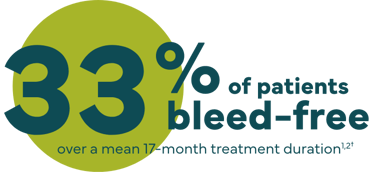
*Previously treated patients (PTPs) receiving routine prophylactic treatment of 35 IU/kg to 75 IU/kg of IXINITY once to twice weekly demonstrated a median (interquartile range [IQR]) annualized spontaneous bleeding rate (AsBR) of 0, and a median total annualized bleeding rate (ABR) of 0.86.1
†The efficacy and safety of IXINITY have been evaluated in a prospective, multicenter, multicountry trial in 21 PTPs (<6 years and 6 to <12 years) who received twice or once weekly prophylaxis treatment with IXINITY for a mean of 159 exposure days.1
Effective control of bleeding episodes
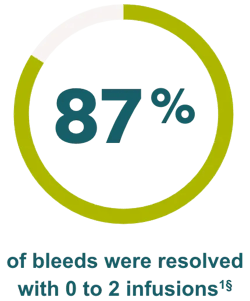
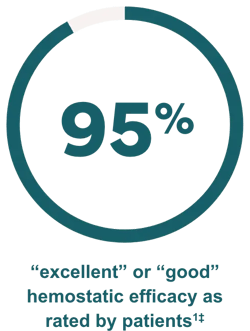
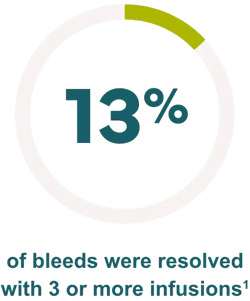
‡The efficacy and safety of IXINITY have been evaluated in a prospective, multicenter, multicountry trial in 21 PTPs (<6 years and 6 to <12 years)1
§In a clinical trial, 17% of bleeding episodes were resolved with 0 infusions, 69% were resolved with 1 or 2 infusions, 8% were resolved with 3 infusions, 4% were resolved with 4 infusions, and 2% were resolved with 5 infusions. A total of 43 bleeding episodes were treated with IXINITY.1
IXINITY clinical trial overview
The efficacy and safety of IXINITY have been evaluated in a prospective, multicenter, multicountry trial of previously treated patients (PTPs)‖ (<6 years and 6 to <12 years) who received IXINITY in a routine regimen.1¶
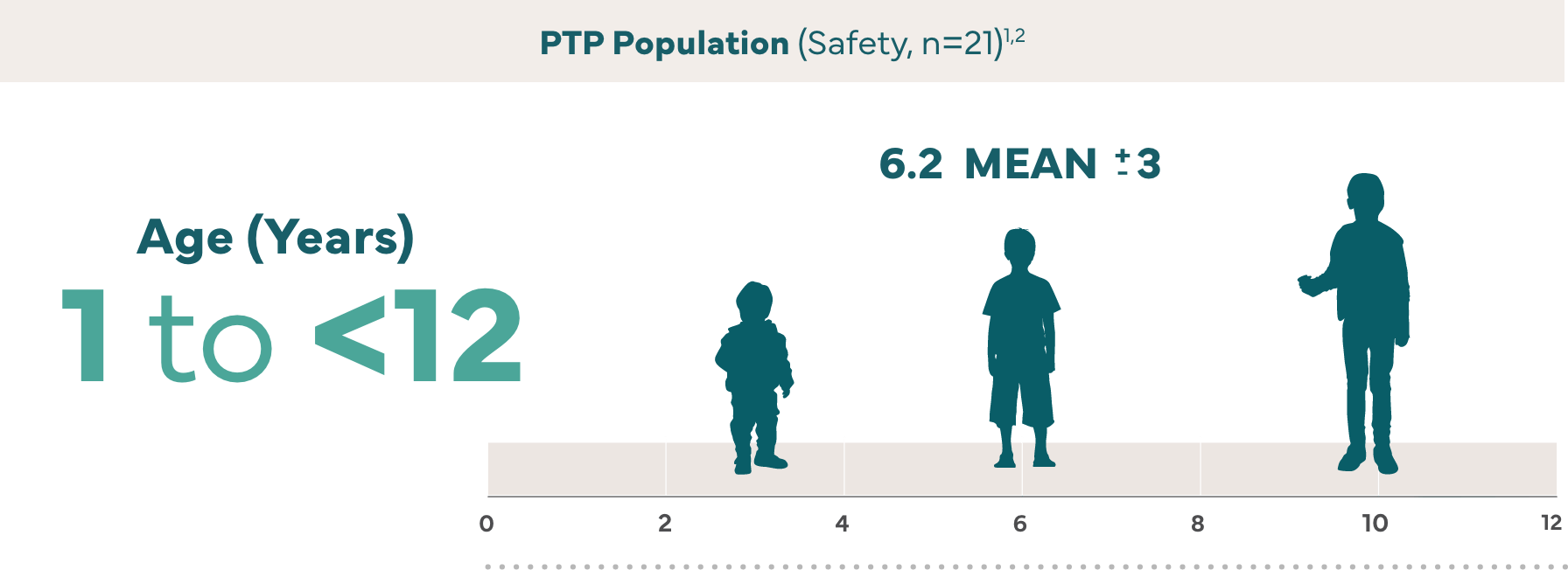

Subjects received twice or once weekly prophylaxis treatment with IXINITY for a mean of 159 exposure days.1
‖PTPs were defined as patients with a minimum of 50 ED to another factor IX preparation. All subjects had severe or moderately severe (factor IX level ≤2 IU/dL) hemophilia B.1
¶Patients received IXINITY prophylaxis once to twice weekly, the recommended dose range was 35 to 75 IU/kg. Subjects < 6 years received a mean intravenous dose of 58 (45-72) ± 8.9 IU/kg and subjects 6 to <12 years of age received a mean intravenous dose of 52 (46-60) ± 11.6 IU/kg. The mean number of ED was 159 (median 163), including 16 subjects with ≥100 ED and 7 subjects with ≥200 ED.1
Discover the clinical trial results of IXINITY in adult and adolescent patients
See the results >References: 1. IXINITY [coagulation factor IX (recombinant)]. Prescribing information. Chicago, IL: Medexus Pharma, Inc.; March 2024. 2. Data on file. Chicago, IL: Medexus Pharma, Inc.



