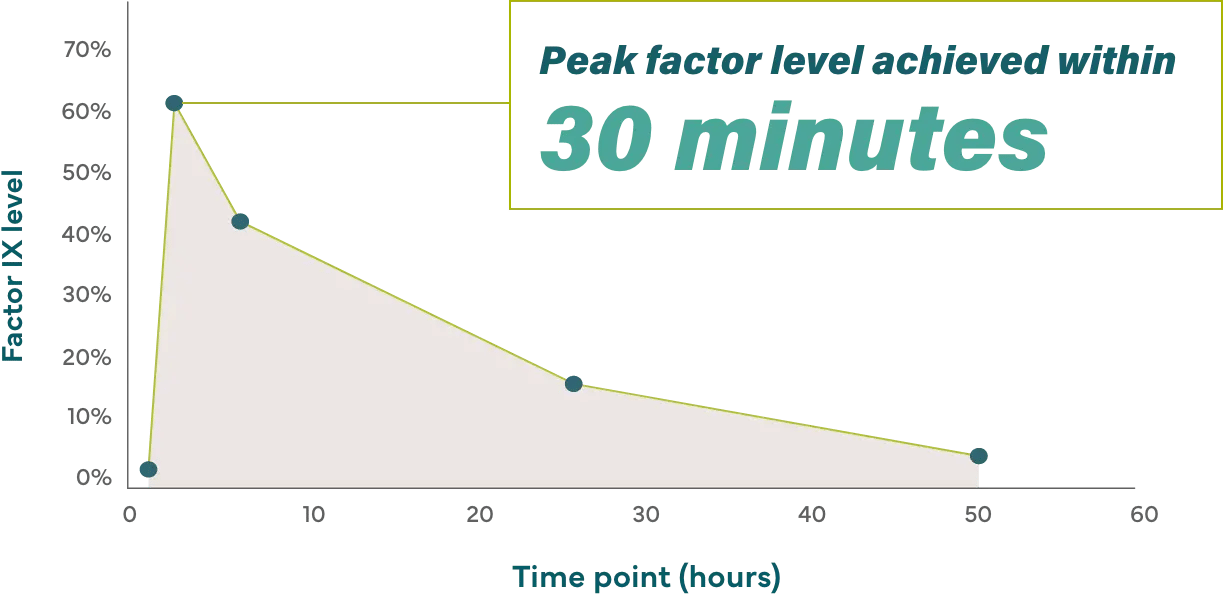
For pediatric patients under 12 years of age
Go low with IXINITY® to keep factor IX coverage high
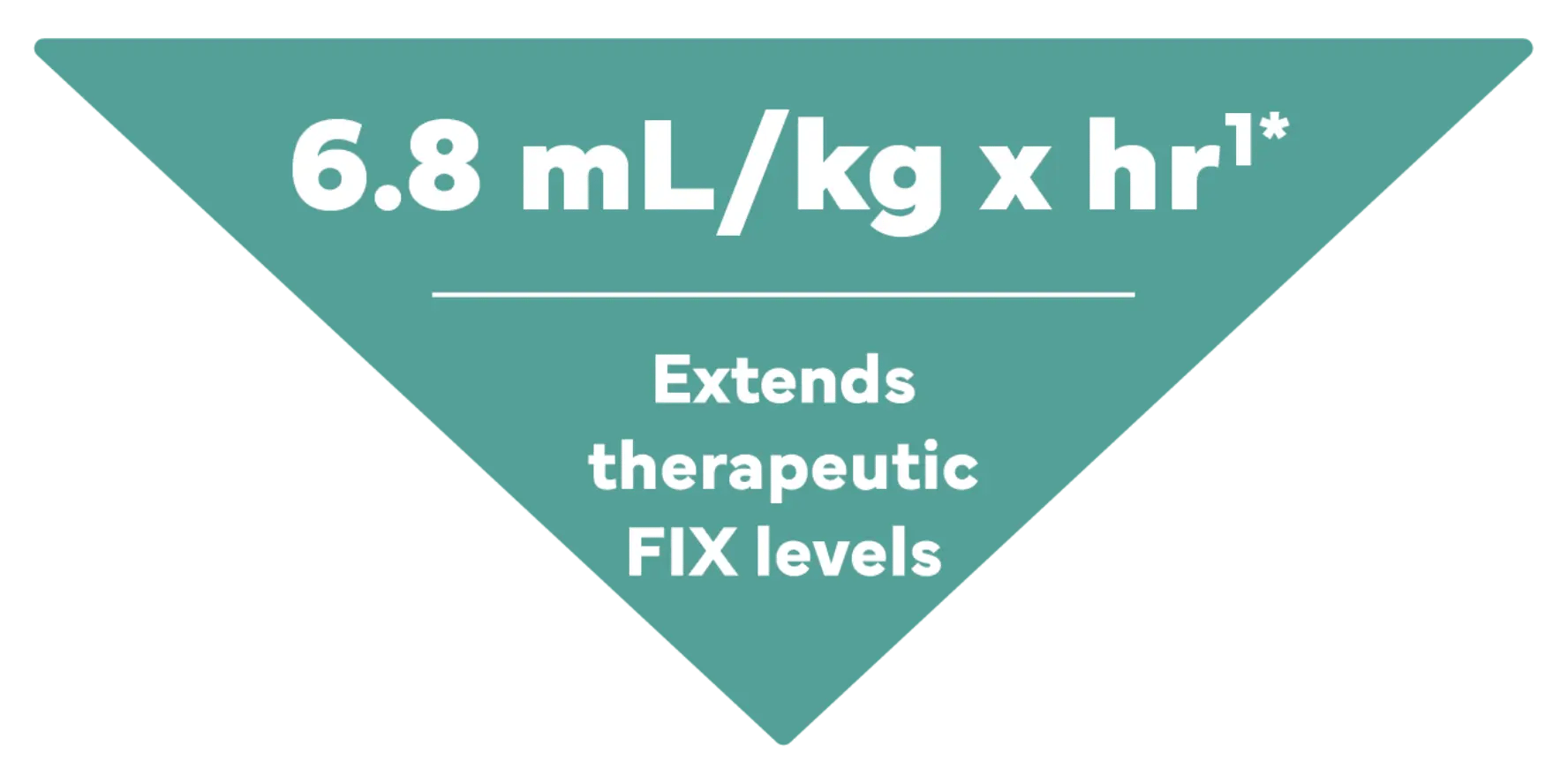
Clearance drives high total exposure to factor
The most robust pharmacokinetic parameter for factor treatment is clearance as it is calculated from the area under the curve (AUC), which shows how well therapeutic factor IX levels are maintained.2
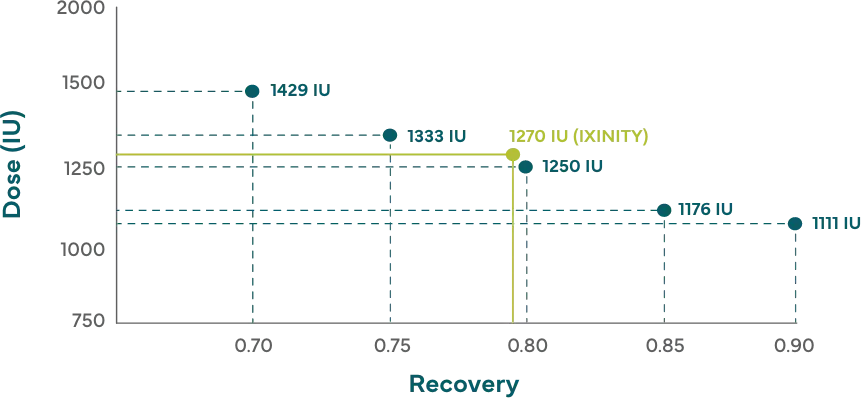
Get more factor IX on board after infusion
Higher recovery with IXINITY may mean more factor IX in the bloodstream

Hypothetical example:
Patient who weighs 20 kg with a desired factor IX increase of 50% and a baseline factor level of <1%†
*The pharmacokinetics of IXINITY have been evaluated in 20 previously treated patients (PTPs) with severe to moderately severe hemophilia B as part of a combined pediatric trial (<6 years and 6 to <12 years of age).1
†Description is not of an actual patient. Individual dose will vary.
FIX = factor IX.

16.3Hour1
high standard
half-life
16.3 hour half-life helps patients achieve peak factor levels when they need them, so they don’t have to worry about if—or when—they’re covered.
Learn about the pharmacokinetics of IXINITY in adult and adolescent patients
Explore The Data >References: 1. IXINITY [coagulation factor IX (recombinant)]. Prescribing information. Chicago, IL: Medexus Pharma, Inc.; March 2024. 2. Collins PW, Quon DVK, Makris M, et al. Pharmacokinetics, safety and efficacy of a recombinant factor IX product, trenonacog alfa in previously treated haemophilia B patients. Haemophilia. 2018;24:104-112. 3. Data on file. Chicago, IL: Medexus Pharma, Inc.