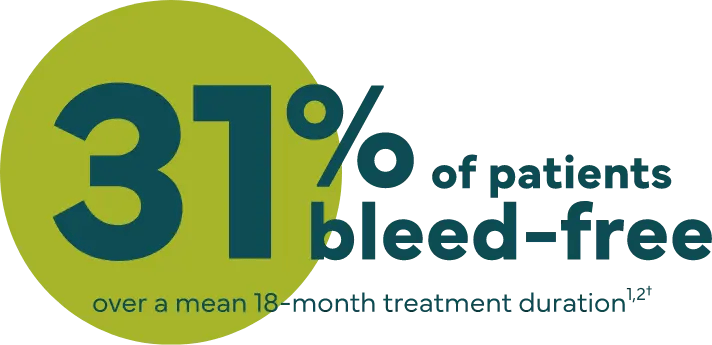
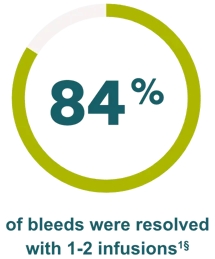
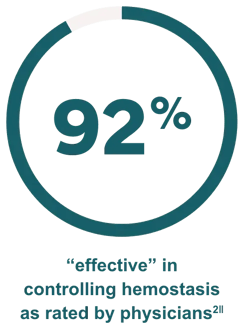
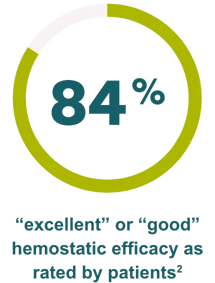
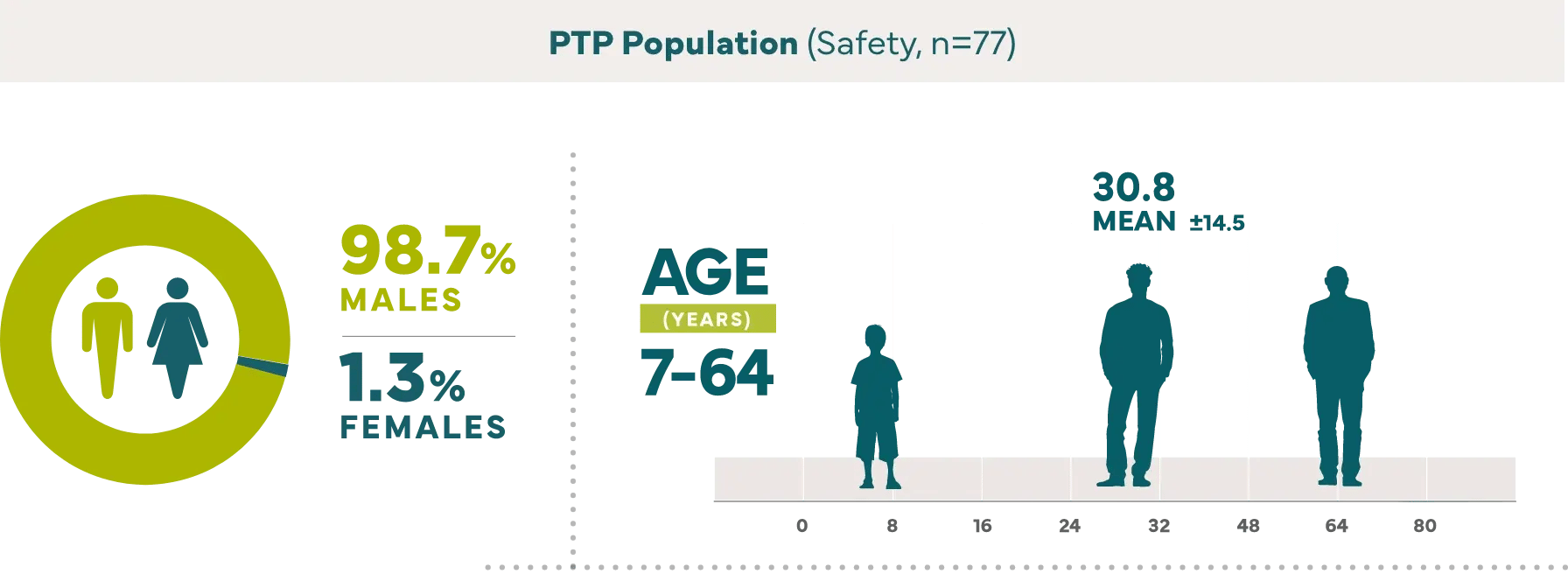
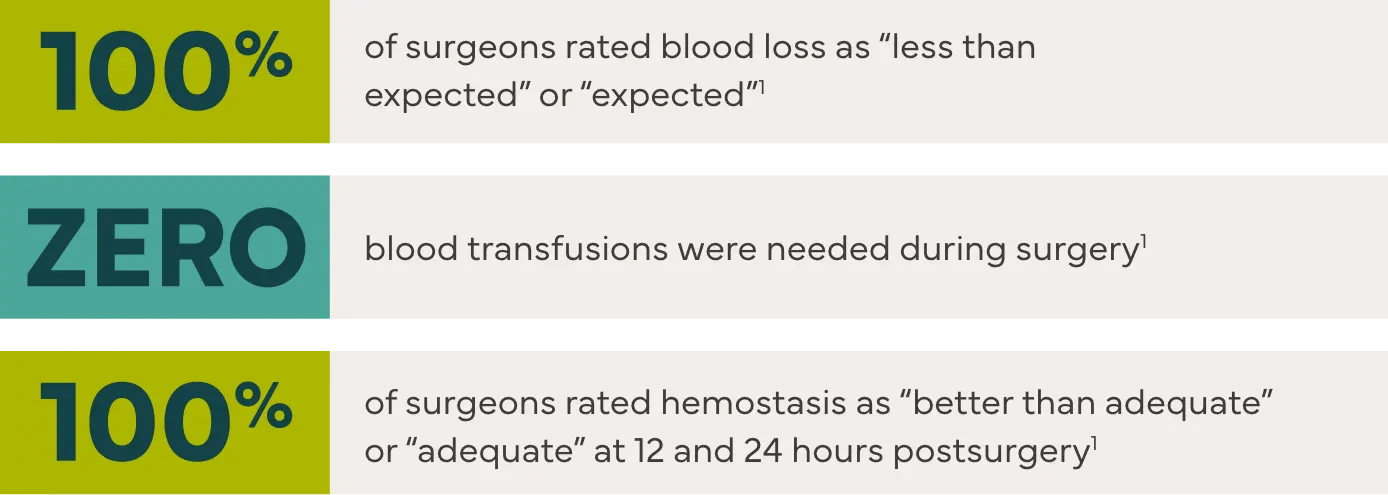
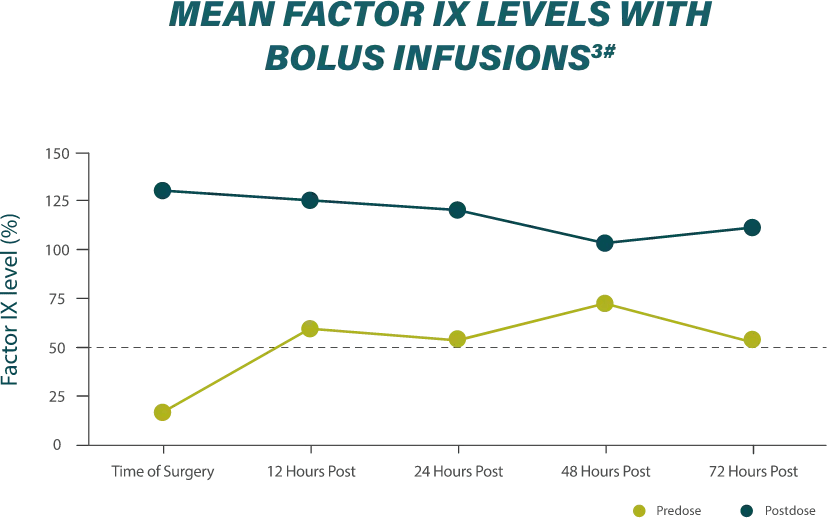
Effective hemostasis was reported with the following major surgical procedures1:
- Knee and elbow arthroplasty
- Knee amputation
- Percutaneous Achilles tendon lengthening
- Arthroscopic synovectomy
- Debridement (ankle, knee)
- Open inguinal hernia repair
- Tibiotalar fusion